Busting Myths: Part 3
Vaccine safety: Three (scientific) reasons why they are safe
With the impending mass COVID19 vaccination rollout in Sri Lanka, there are murmurs about vaccines causing allergic reactions or even deaths. It was only with its mass vaccination programs that Sri Lanka could eradicate diseases like measles or polio. It remains integral to economic recovery that there is public buy-in for a COVID19 vaccination scheme.
To that end, we break down the results from the clinical trials to see whether these rumours have legs or are just in fact, misinformation.
How effective are these vaccines? Little recap from Part 1 of our Blog Series
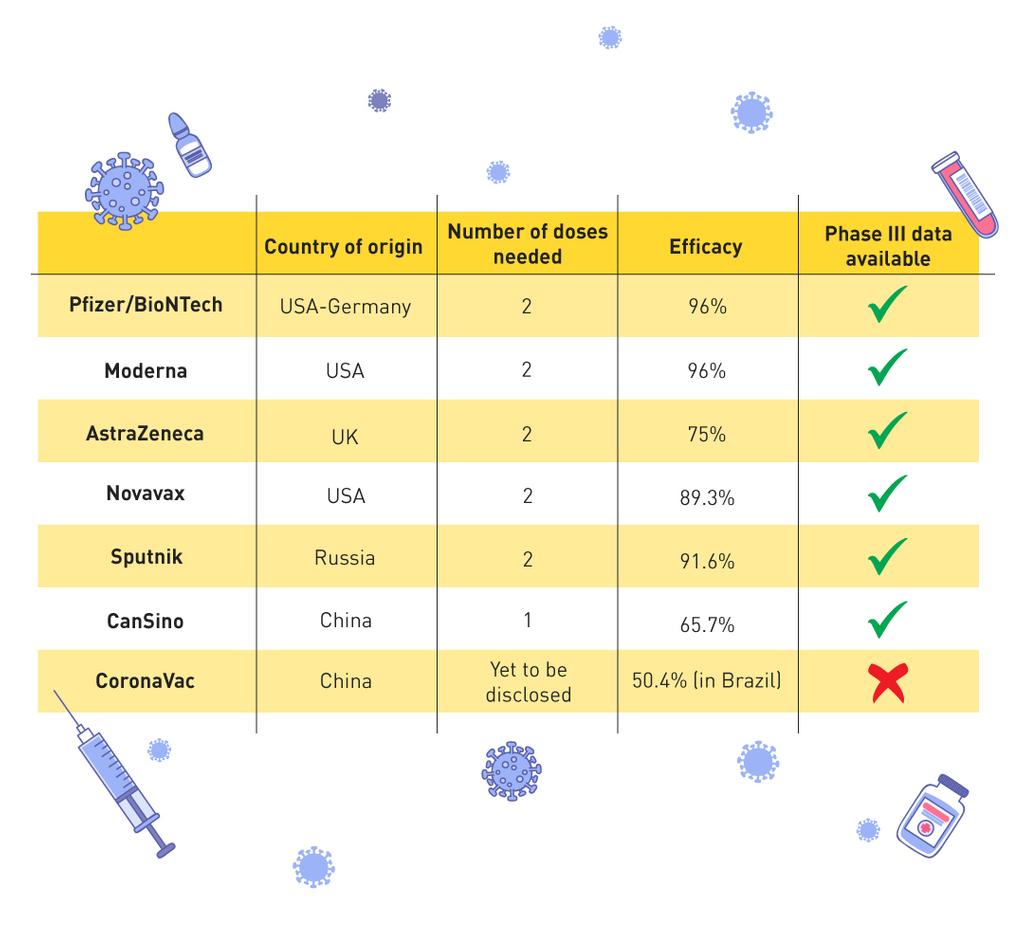
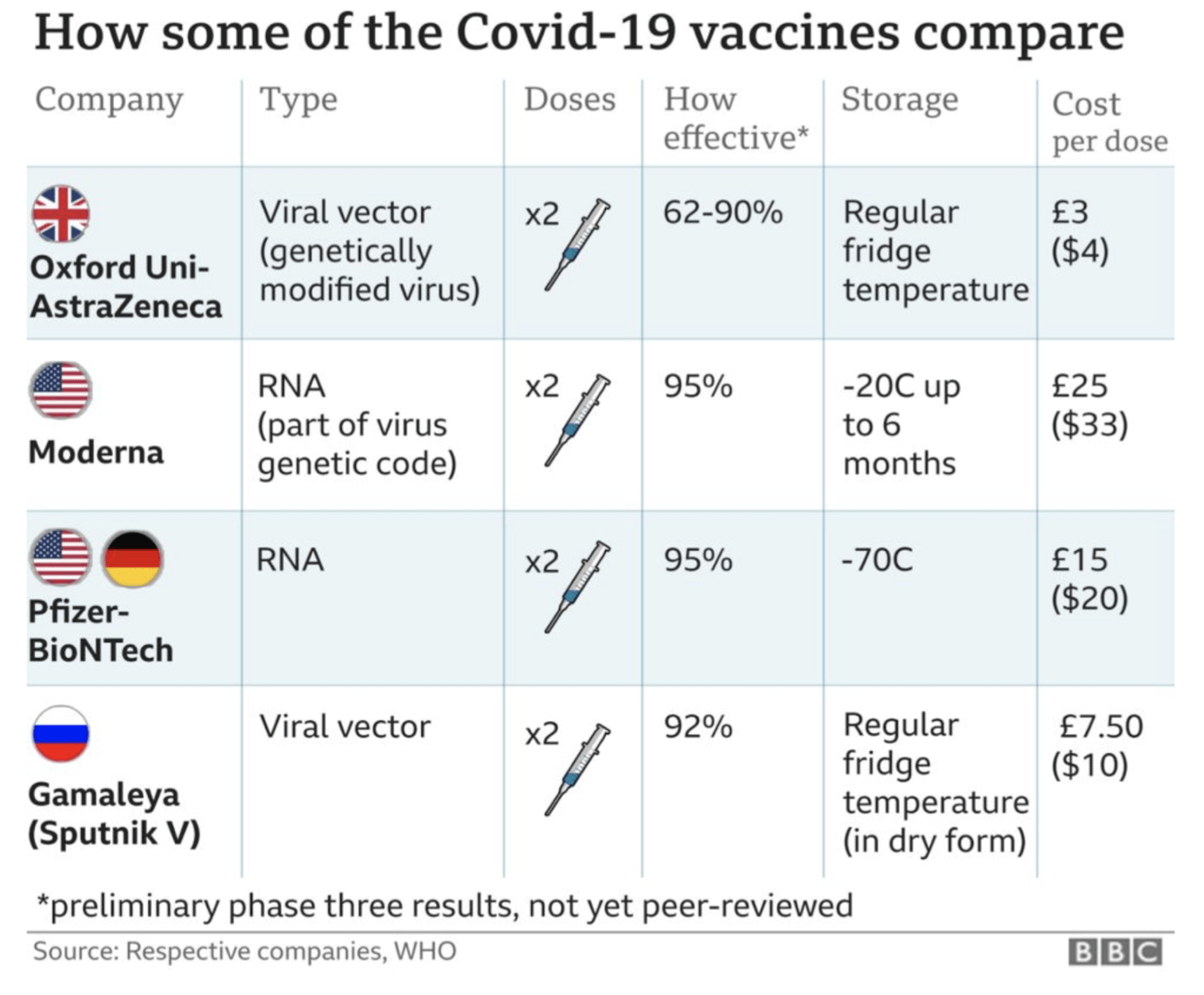
Clinical trial data has shown that all of the vaccines authorised so far are very effective at preventing symptomatic illness. The table below gives a quick summary of the findings so far:
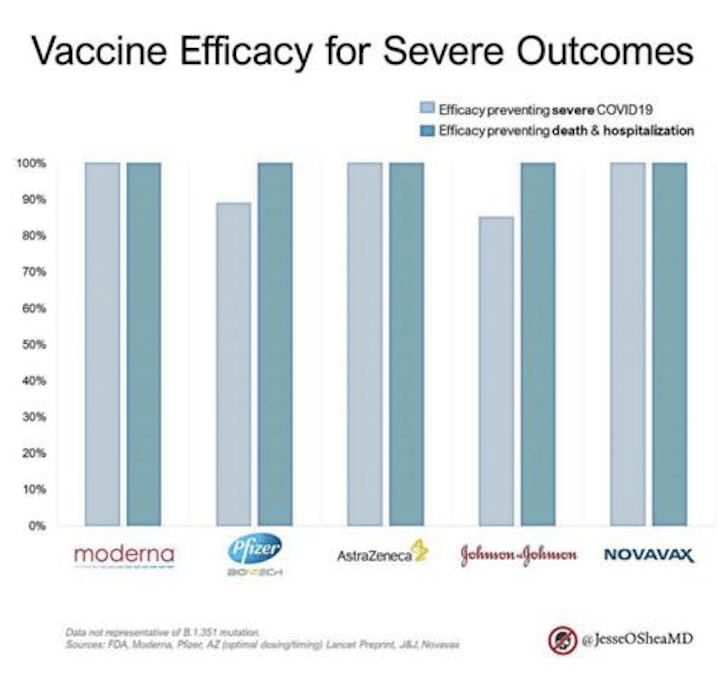
Importantly ALL the vaccines have 80%+ efficacy against preventing severe disease and 100% against deaths & hospitalisation. After 2.3million deaths around the world, this is welcome news.
1) The trials have 30-40,000 people each to mimic real-world conditions.
Phase III clinical trials are the most extensive, most time consuming and most expensive part of the development process. Scientists look to mimic real-world conditions (different ages, genders, cities etc.). They aim to see whether the vaccine effectively protects against the virus AND whether it causes any side effects in the wider population.
AstraZeneca & Moderna enrolled 30,000 people each in their Phase III, Pfizer/BioNTech 42,000, Novavax 20,000 and Russia’s Sputnik 21,000. In comparison, the GlaxoSmithKline MMR vaccine Phase III trial only enrolled 5,000 participants and is now a widely accepted part of the Sri Lankan immunisation strategy(3).
Sample sizes are large to reduce “sampling error”. Sampling error is the difference between the sampled results and the population’s results if the sample doesn’t represent the population accurately. By enrolling tens of thousands of people – each of these vaccine trials could reduce sampling error and thus be as accurate as possible in their data collection.
2) The trials showed mild reactions in mostly younger people which lasted a median of one day.
Every vaccine trial looks for occurrences of “local” (at the site of injection) and “systemic” (around the body) reactions to the vaccine. Local reactions include injection site pain, redness, swelling. Systemic adverse reactions include fever, fatigue, headache, chills, vomiting, diarrhoea, muscle or joint pain. If reactions occur, they happen within 1-2 days of getting the vaccine and last an average of one day.
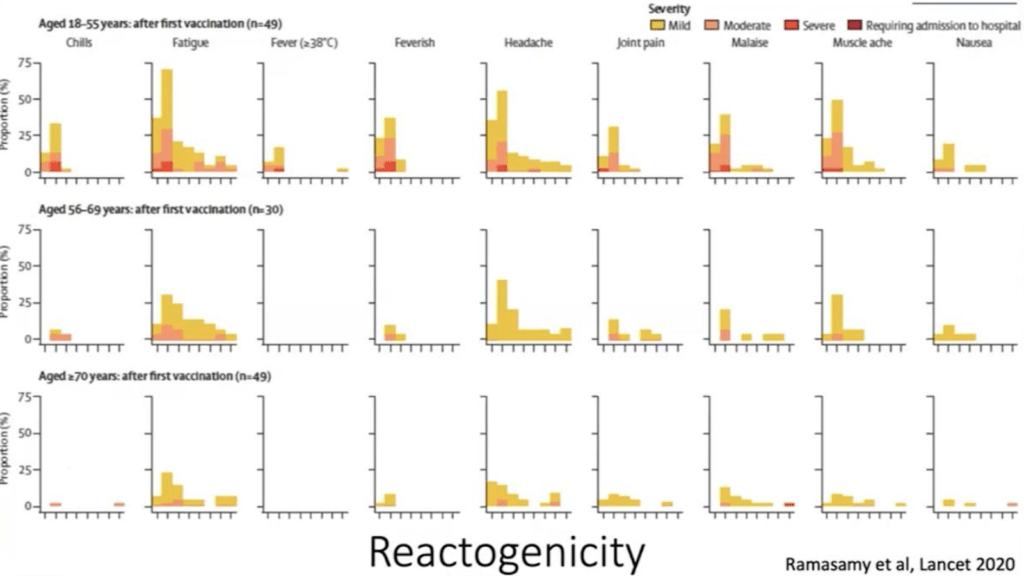
AstraZeneca separated its participants into three age groups: 18-55, 56-69, and 70+. In the table below, we can see which local and systemic reactions occurred in the study participants and how long they lasted.
They were more common in the 18-55 age group with most complaints of fatigue, headache and muscle ache soon after getting the vaccine. For all age groups and all complaints, the reactions disappeared in a few days(4).
Pfizer/BioNTech saw younger people experiencing local and systemic adverse reactions more often and in greater intensity than the older age groups. They often felt pain at the injection site at a greater intensity than the older participants. Both groups complained of fatigue, headache and body aches.
All of Moderna’s participants complained of local reactions with pain & temporary swelling of lymph nodes being the most common. However, most reactions only lasted 1 to 2 days. Fatigue was the most commonly reported systemic reaction followed by headaches, body ache, fever and chills for 1-2 days post-vaccination.
3) The vaccines rarely caused severe adverse reactions and showed to be 100% protection against death.
As Phase III is designed to mimic the real population, events such as deaths or heart attacks that will typically occur in the real population also occur in the trial groups. The safety boards’ responsibility is to review the data to decide which events were caused by the vaccine and which occurred naturally. For this, they look at rates of these events in the general population by age group and deep dive into each case’s particulars.
None of the vaccine trials (including Russia’s Sputnik) has had a severe adverse effect resulting in death as a result of the vaccines (5)(6). Of the 84 severe adverse events (in 11,000 candidates) reported by AstraZeneca, only one classified as possibly related to the vaccine(4). This one case is 0.000085% of the total study population, a tiny percentage.
All of the vaccines peer-reviewed so far shows to have 100% efficacy against death & hospitalisation.
Weighing up the benefits
COVID19 has caused 2.3million deaths around the world(8) and over 300 deaths in Sri Lanka. None of the COVID19 vaccines reviewed thus far has yet had a death determined to result from the vaccine. Adverse events are most likely to occur soon after vaccine administration. With over 128 million doses being administered globally, there have been less than 0.005% reports of severe adverse reactions.
The choice is between contracting a virus known to cause lasting damage to the body (if not death) and taking a vaccine that is found to prevent death 100% of the time. Moreover, the vaccines reviewed thus far are not known to cause severe adverse events; the winner becomes abundantly clear.
We would urge the Sri Lankan public to accept the AstraZeneca or Pfizer vaccines.
Read our previous blog on Do the vaccines actually work?
Sources
- AstraZeneca Press Release (Sept 20) Statement on AstraZeneca Oxford SARS-CoV-2 vaccine, AZD1222, COVID-19 vaccine trials temporary pause.
- AstraZeneca Press Release, (Sept 20) COVID-19 vaccine AZD1222 clinical trials resumed in the UK.
- GlaxoSmithKline (2012) Consistency Study of GlaxoSmithKline (GSK) Biologicals’ MMR Vaccine (209762) (Priorix) Comparing Immunogenicity and Safety to Merck & Co., Inc.’s MMR Vaccine (M-M-R II), in Children 12 to 15 Months of Age. Clinical Trials.gov
- Ramasamy et al (2020)., Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial. The Lancet 396: 1979-1993.
- Voysey et al (2020), Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. The Lancet 399: 99-111.
- Pfizer-BioNTech (2020).,Vaccines and Related Biological Products Advisory Committee Meeting., FDA Briefing Document.
- Moderna (2020).,Vaccines and Related Biological Products Advisory Committee Meeting., FDA Briefing Document.
- Logunov. D et al (2021)., Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. The Lancet. Online first.
- Bloomberg COVID19 Vaccine tracker
- Dr. Widmer (2021) Fact-check: No link between COVID-19 vaccines and those who die after receiving them. ABC News.
- Primary Care Pharmacy Network., Delivery of COVID Vaccination Services (2020)
- Allergic Reactions Including Anaphylaxis After Receipt of the First Dose of Pfizer-BioNTech COVID-19 Vaccine — United States, CDC, December 14–23, 2020
- COVID19 Vaccines and Allergic Reactions, CDC, January 2021
- Moderna: FACT SHEET FOR RECIPIENTS AND CAREGIVERS, FDA
- Information for UK recipients on COVID 19 Vaccine AstraZeneca, MHRA
Similar Articles...

Let’s talk flu, its prevention and home remedies.
Boo-ger season is here! Let’s begin by defining flu (short term for influenza) because it’s usually misunderstood as fever or cold. Flu is a common

What’s The Deal With COVID19 Boosters?
As Sri Lanka rolls out its COVID19 booster program, we break down the answers to your most pressing questions. Firstly, what is a booster? A

Back to School – A Battle Between Education and COVID-19
Back to School – A Battle Between Education and COVID-19 Students are finally returning to school. But as parents, many are worried about COVID-19 safety.